Possible Microbiome Therapies In Me/cfs Patients
Although multiple studies show evidence that inflammation in the gut as well as several diseases are associated with an altered gut-brain communication, evidence for a successful therapy in response to these complaints is rare . Two widely discussed therapies are probiotic interventions and fecal microbiota transplantation .
3.8.1 Probiotic Interventions
The idea of changing the intestinal microbiota, enhancing health and preventing diseases by the intake of beneficial live microorganisms sounds obvious and has not only attracted the attention of researchers and gastroenterologists, but also industry and marketing experts . A meta-analysis with 1793 patients came to the conclusion that probiotic use is effective for pain and symptom severity management in IBS patients . In contrast, a systematic review over all published probiotic meta-analyses concluded that a benefit of probiotics was only evident for antibiotic- and Clostridium difficile-associated diarrhea and respiratory tract infections .
3.8.2 Fecal Microbiota Transplantation in ME/CFS Patients
3.8.3 Fasting Dietary Interventions
Impaired immune cell mitochondria and the microbiome were recently suggested to interact with circadian rhythm and driving ME/CFS pathophysiology .
3.8.4 Other Potentially Gut Microbiome Mediated Approaches
What Is C Diff
Anyone can get sick from C. diff bacteria. C. diff makes spores that are shed in feces and can live on surfaces for up to five months, even after cleaning with disinfectants. Once on your hands, the bacterium enters your body through your mouth and travels through your digestive tract to your intestines .
The most common symptoms of a C. diff infection are:
- Having had surgery involving the intestines
What About The Human Genome
In a study of aortic aneurysms, Gottlieb et al. reported that BAK1 SNP-containing alleles were detected in aortic tissue but not in blood samples from the same patients. More recently Ursini et al. found that schizophrenia gene risk loci that interact with early-life complications are highly expressed in the placenta. However, these loci were differentially expressed in placentas from women who suffered complications during pregnancy. They were also differentially upregulated in placentae from male compared with female offspring.
These and related findings strongly suggest that the environment can select for human genome activity. For example, Harley et al. found that in EBV infected cells, EBNA2 and its transcription factors modulated the activity of human genes associated with risk for multiple sclerosis, rheumatoid arthritis, type 1 diabetes and other conditions. In fact, nearly half of systemic lupus erythematosus risk loci were occupied by EBNA2 and co-clustering human transcription factors.
Studies of the human genome must also account for the full extent of microbial DNA and RNA in human tissue and blood. If a genomic assembler fails to account for this contamination, chances of a false positive single nucleotide polymorphism increase significantly during analysis . Contamination with even a small amount of microbial DNA/RNAjust one or two base pairs of differenceis enough to cause significant statistical errors in this fashion.
You May Like: How To Help Eye Fatigue
Therapeutic Restoration Of Mucosal Barrier Function
Since altered intestinal microbiota and gut barrier dysfunction barrier are found in CFS , they offer potential targets for intervention that would include modulation of the gut microbiota to correct an imbalance, as well as tightening of interepithelial junctions. Enhancement of barrier function by probiotic bacteria has been observed in both in vitro models and in vivo animal models .
Probiotics are live microorganisms with a vast array of therapeutic potential for GI disease. They have a beneficial effect on the intestinal mucosa via several proposed mechanisms that include inhibition of the mucosal adhesion of pathogens, improvement of the barrier function of the epithelium, and alteration of the immune activity of the host. They may also regulate intraluminal fermentation and stabilize the gut microbiota . In addition, probiotics have recently emerged as promising adjunctive therapy in treating IBS, with B. infantis becoming the frontrunner for treatment .
Bifidobacteria appear to play an important role in maintaining the gut barrier. An increase in Bifidobacteria in ob/ob mice was associated with a significant improvement of gut permeability measured in vivo this improvement was linked to an increase in tight junction mRNA expression and protein distribution . In addition, the rise in Bifidobacteria was correlated with a decrease in plasma LPS concentrations therefore, a significant reduction in markers of oxidative and inflammatory stress .
Precautions And Possible Complications

Its a good idea to get tested for C. diff if you exhibit symptoms, especially if symptoms occur during or after taking antibiotics or after being around someone that you know has a C. diff infection.
What complications can C. diff cause? If the infection worsens, you may become seriously dehydrated, be unable to pass stool and/or experience weight loss. C. diff can also lead to a toxic megacolon, which may require emergency surgery or bowel perforation, which can lead to a dangerous infection called peritonitis. Can C. diff kill you? Rarely, a C. diff infection can lead to a hole in the intestines or , which can be deadly. In severe cases, surgery may be required to remove the infected part of the colon.
Can you kiss someone with C. diff? Its usually considered OK to kiss and hug someone with C. diff since the infection is not typically spread through touching, and it also is not spread through the air by things like sneezing or coughing. However, if you visit someone hospitalized with C. diff, you should take some key C. diff precautions, including wearing gloves while in the room and washing your hands before leaving their room. Wearing gloves and practicing good hand washing hygiene is also very important for people who work in hospitals and long-term care facilities.
Read Also: How To Fight Fatigue Naturally
Looking After Yourself At Home
If you’re well enough to be treated at home, the following measures can help relieve your symptoms and prevent the infection spreading:
- make sure you finish the entire course of any antibiotics you’re prescribed, even if you’re feeling better
- drink plenty of fluids to avoid dehydration and eat plain foods such as soup, rice, pasta and bread if you feel hungry
- take paracetamol for tummy pain or a fever
- don’t take anti-diarrhoeal medication, as this can stop the infection being cleared from your body
- regularly wash your hands and contaminated surfaces, objects or sheets
- stay at home until at least 48 hours after your last episode of diarrhoea
Your GP may contact you regularly to make sure you’re getting better. Call them if your symptoms return after treatment finishes, as it may need to be repeated.
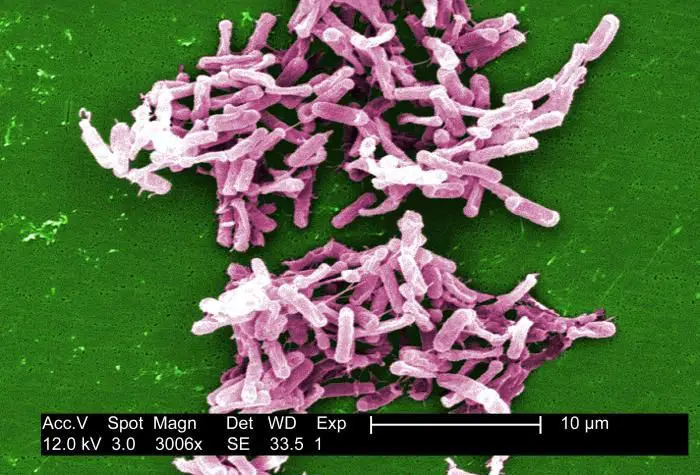
Complications Of C Diff
C. diff
- Dehydration
- Toxic megacolon
The severe diarrhea caused by C. diff can lead to a significant loss of fluids and electrolytes, making it difficult for your body to function normally. In turn, this can cause blood pressure to drop to dangerously low levels.
In some cases of C. diff infection, dehydration occurs so rapidly that kidney function deteriorates, leading to kidney failure.
In addition, toxic megacolon is a rare condition that can develop after C. diff infection. If your colon is unable to expel gas and stool, it can become greatly distended, potentially causing it to rupture and allow bacteria to seep into your abdominal cavity .
Toxic megacolon requires emergency surgery and can be fatal if left untreated. A bowel perforation, or hole in your large intestine, is a rare complication that results from extensive damage to the lining of the organ following toxic megacolon.
C. diffC. diff
Also Check: Nicole Miller Anti Fatigue Mat
How Is C Diff Diagnosed
Doctors will suspect C. diff infection when a person develops diarrhea within two months of using an antibiotic. A lab test confirms the diagnosis by looking for one of the toxins produced by C. diff in a stool sample.
Imaging tests such as abdominal X-rays or computed tomography may be done if a serious complication is suspected.
Past Antibiotic Intake Hypothesis In Me/cfs Patients
It is well known that the postnatal and early life use of antibiotics disrupts the neonatal microbiome and is associated with a higher risk of several diseases such as asthma, IBD or type 2 diabetes . Also in adults there is, for example, an increased risk of 13% to develop diabetes after an antibiotic treatment , which supports that changes in the microbiome alter some metabolic conditions. According to a very large retrospective study, one treatment with antibiotics also increases the risk of developing IBD by 84% and with every following treatment there is a 6% higher risk. The researchers were also able to show that the earlier and more frequently antibiotics were taken, the higher was the risk of IBD . IBD is also often mentioned in connection with ME/CFS, although the correlation remains unclear . Interestingly, the mode of delivery influences the amount of antibiotic resistance genes in the microbiome, with vaginally delivered infants appearing to be relatively protected against antibiotic resistance . Berstad and co-workers suggested with their three-set dysbiotic march hypothesis an explanation for the high comorbidity in ME/CFS patients with IBS, as described above .
After the discovery of penicillin, antibiotics massively intruded into medical practice in the 1940s and 1960s . According to a recent report, antibiotics disrupt the bacterial composition in the gut for up to 4 years and to some extent permanently .
Read Also: How Do You Test For Adrenal Fatigue
Natural C Diff Treatment
Can C. difficile go away on its own? According to some medical doctors, the infection can go away on its own and sometimes people are less likely to have a recurrence if not treated. However, even mild C. diff symptoms mean that your system is clearly off and could use a boost. If youre wondering how to treat C. diff at home, there are a lot things you can do to help fight off this intestinal infection. The following are some of the natural approaches, including an anti-C. diff diet, that can really help to fight an infection.
1. Stop Antibiotics Whenever Possible
Natural as well as conventional C. diff guidelines include stopping any antibiotic medications you are currently taking. Even the CDC recommends that one of the first things you want to do when you find out you have a C. diff infection is to stop taking any antibiotics you are currently on whenever possible. These antibiotics are killing off the good bacteria in your body, and this is exactly what C. diff bacteria wants they want the good bacteria decreased so they can overgrow and take over. Stopping other antibiotics can provide noticeable improvement in C. diff symptoms, especially diarrhea, quite quickly. Be sure to consult with your healthcare practitioner before stopping medications, however.
2. Load Up On Good Bacteria
3. Avoid or Reduce Certain Foods
What foods should be avoided with C. diff? According to the C. Diff Foundation, avoiding the following foods can be helpful when fighting an infection:
Which Infection Causes Cfs & Fibromyalgia
The question of which infection, if any, is the cause of CFS and fibromyalgia has been both the Holy Grail and bane of CFS/fibromyalgia researchers. Literally dozens of infections have been implicated, with virtually all of them failing to have reliable testing available to confirm the infection. Because of this, taking a good clinical history, and looking for the response to treatment, continues to be the best approach.
So which infection is the cause of CFS and fibromyalgia? The simple answer is that it isn’t a specific infection, but rather an immune dysfunction. This allows many opportunistic infections, ones that cannot survive in a healthy immune system, to cause problems.
Our impression is that the body shows many areas of immune dysfunction, including the inability to turn off the immune system even after certain common viral infections have been eliminated. This contributes to overactivity, followed by exhaustion, of the immune system, resulting in multiple “hitchhiker” infections being present.
It isn’t necessary to treat every infection. But some do need to be addressed. The most important: Candida overgrowth. There is no test that I find reliable for this, but I treat most people who have CFS and fibromyalgia with six weeks of the medication Diflucan 200 mg a day, combined with a low-sugar diet and a weaker antifungal to decrease the risk of developing resistant strains of Candida.
Recommended Reading: Best Industrial Anti Fatigue Mat
Reduced Gut Bacteria Diversity Anti
To reach their findings, the researchers analyzed the stool and blood samples of 48 people who had been diagnosed with CFS, alongside the samples of 39 healthy controls.
Compared with the stool samples from the healthy controls, the stool samples from CFS patients showed reduced gut bacteria diversity, fewer anti-inflammatory bacteria, and more pro-inflammatory bacteria.
- Women are two to four times more likely to develop CFS than men
- While children can develop CFS, it is much less common than for adults
- There are currently no treatments for CFS that have been approved by the Food and Drug Administration .
The team notes that such abnormalities in gut bacteria are often seen in the stool samples of patients with Crohns disease and ulcerative colitis, which are inflammatory bowel diseases.
Additionally, the researchers found that the blood samples of patients with CFS contained markers of inflammation. They say this is likely a result of bacteria entering the blood due to a leaky gut, which has been triggered by intestinal problems.
The team explains that when such bacteria enter the blood, this can set off an immune response, which could exacerbate symptoms of CFS.
Using the newly obtained information from stool and blood samples, the researchers found they could correctly diagnose CFS in 83 percent of patients a result that could pave the way for new diagnostic and treatment methods for the condition.
Signs Of Chronic Fatigue Found In Gut Bacteria
You are free to share this article under the Attribution 4.0 International license.
Chronic fatigue syndrome, a condition where normal exertion leads to debilitating fatigue that isnt alleviated by rest, has long mystified scientists. There are no known triggers, and diagnosis requires lengthy tests.
Some have suggested the disease may be psychosomatic.
Now, for the first time, researchers report they have identified biological markers of the disease in gut bacteria and inflammatory microbial agents in the blood.
In a new study published in the journal Microbiome, scientists describe how they correctly diagnosed myalgic encephalomyeletis/chronic fatigue syndrome in 83 percent of patients through stool samples and blood work, offering a noninvasive diagnosis and taking a step toward understanding the cause of the disease.
Our work demonstrates that the gut bacterial microbiome in ME/CFS patients isnt normal, perhaps leading to gastrointestinal and inflammatory symptoms in victims of the disease, says lead author Maureen Hanson, professor of molecular biology and genetics. Furthermore, our detection of a biological abnormality provides further evidence against the ridiculous concept that the disease is psychological in origin.
Thats very typical and specific of people with ME/CFS, because healthy people, or even people who have heart disease, can reproduce the exercise on the second day, but these people cannot, Giloteaux says.
Read Also: What Can You Do To Combat Fatigue
Fecal Matter Transplant Treatment
A newer and more highly effective treatment is a fecal matter transplant, where the fecal matter from a person with healthy gut flora is mixed with saline, strained, inserted into the patient with Clostridium difficile colitis via a colonoscopy, endoscopy, sigmoidoscopy, or enema in order to recolonize the ill person’s bowels.
Chronic Fatigue Syndrome: Could Altered Gut Bacteria Be A Cause
What causes chronic fatigue syndrome? The answer to this question continues to baffle researchers, so much so that some have even questioned whether the condition exists. Now, a new study by researchers from Cornell University in Ithaca, NY, may have shed light on a biological cause, after finding that patients with chronic fatigue have an altered gut microbiome.
Senior author Maureen Hanson, of the Departments of Molecular Biology and Genetics and Microbiology at Cornell, and colleagues publish their findings in the journal Microbiome.
Also referred to as myalgic encephalomyelitis , chronic fatigue syndrome is a condition characterized by extreme fatigue that does not improve with rest.
Aside from persistent fatigue, symptoms used to diagnose ME/CFS include unrefreshing sleep, headache, joint pain, sore throat, tender lymph nodes in the neck or armpits, problems with concentration and memory, and severe exhaustion and sickness after exercise or mental exertion.
A diagnosis of CFS may be made if four or more of these symptoms last 6 months or longer.
Other symptoms of the condition may include visual problems, dizziness or fainting, brain fog, and irritable bowel.
Because the symptoms of CFS are very similar to those of other illnesses, the condition can be tricky to diagnose. As such, it is unclear how many people in the United States have CFS, though estimates suggest it affects around .
Also Check: What Type Of Doctor Treats Chronic Fatigue Syndrome
Different Pathogens Employ Common Survival Strategies
Many pathogens employ common survival mechanisms to persist in host cells, tissue and blood. The metabolic dysfunction driven by these different microbes and viruses can result in similar clusters of human inflammatory symptoms. The ability of various pathogens to dysregulate activity of the Vitamin D Nuclear Receptor is an excellent example of how different microbes can drive similar disease processes. The VDR regulates expression of hundreds of human genes, many of which regulate inflammatory and malignant processes . The receptor also controls signaling of TLR2 and several families of antimicrobial peptides including cathelicidin . Pathogens capable of slowing VDR activity can subsequently facilitate their survival by slowing the innate immune response.
Pathogens frequently linked to ME/CFS or inflammatory disease have evolved to survive in this fashion. VDR activity is downregulated as much as 20 times in Epstein Barr Virus-infected lymphoblastoid cell lines .4). HIV, M. tuberculosis Cytomegalovirus, Borrelia burgdorferi and Mycobacterium leprae additionally dysregulate VDR activity to various degrees . The fungus Aspergillus fumigatus secretes a gliotoxin that significantly downregulates VDR expression . Because disabling the innate immune system via the VDR pathway is such a logical survival mechanism, other uncharacterized bacteria, viruses or fungi may have also evolved to dysregulate receptor activity.